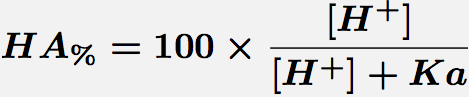
Equation 1 Fraction present as HA
Comments Monoprotic acids, such as acetic or salicylic acid, have one pKa value and thus two species at various pH values. These forms are the free acid form, HA, and the salt form, A-. The concentration of each species can be calculated with the equations.
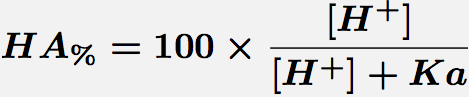
Equation 1 Fraction present as HA
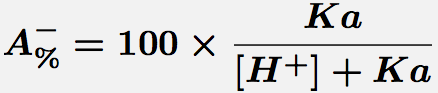
Equation 2 Fraction present as A-
| Acid | pKa1 |
| Acetic* | 4.76 |
| Boric | 9.24 |
| Lactic | 3.86 |
| Salicylic | 2.97 |