




![]()
(Equation IV-2 from earlier in this Chapter)
to calculate kel. Plotting - dCp/dt versus Cp would give kel as the slope of the straight line. Howver, in practice, it is difficult to measure dCp/dt (the tangent to the line drawn through the Cp versus time plot) accurately and so this equation is not often used directly (see Figure 7 from earlier in this Chapter).
The problem is the differential term. Mathematically, we get rid of this term by integrating the equation. We can go through this one because it is easy and also to see what is involved in the process, but for other differential equations we'll just use the answer.
Starting with
![]()
(Equation IV-2 from earlier)
Rearranging gives
![]()
Equation IV-3. Differential Equation Rearranged
Integrating both sides gives
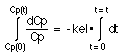
Equation IV-4. Integration of the Rearranged Equation
This gives (by looking the answer in Maths tables) -
![]()
Equation IV-5. Rearrange the Integrated Equation
Note that the integral of 1/x is ln(x), when ln represents log to the base e. This leads to the utility of the log to base e which is used extensively in the area of pharmacokinetics.
Expanding Equation IV-5 gives
ln Cpt - ln Cp0 = - kel * t + kel * 0
Equation IV-6. Natural log of Cp versus Time
Or
ln Cpt = ln Cp0 - kel * t
Equation IV-7. Integrated Equation for ln Cp versus Time
Note the use of natural log to base e
Rearranging gives
![]()
Equation IV-8. Rearranging the Integrated Equation
Taking the antilog (base e) of both sides gives
![]()
Equation IV-9. Another form of the integrated equation
This is a single exponential equation. The fall in plasma concentration is therefore called monoexponential decay
Looking back at the ln (base e) equation; we can convert that into log (base 10), common logs, by dividing by 2.303 giving
![]()
Equation IV-10. Log base 10 Cp versus Time
This is another form of the integrated equation
Both of these integrated (logarithmic) forms represent a straight line equation, that is of the form: y = a - m * t with a = intercept and m = slope.
Plotting ln (Cp) versus t should give a straight line with a slope of - kel and an intercept of ln Cp0.
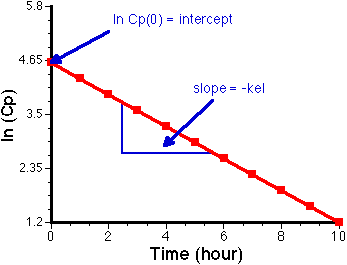
Figure IV-4., Linear plot of Cp versus time, showing intercept and slope. NOTICE, no UNITS for ln (Cp) but units of hour for time (X axis).
Units: Slope has units of time-1 thus kel has units of time-1 e.g. min-1, hr-1.
Now we can measure kel by determining Cp versus time and plotting ln Cp versus time.
On Semi-log graph paper
This plot allows us to calculate kel given Cp at various times.
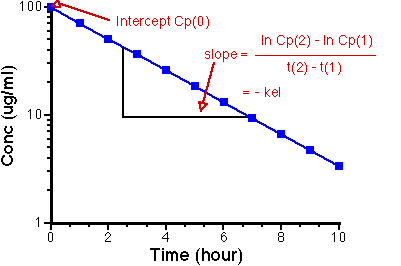
Figure IV-5. Semi-log plot of Cp versus time, showing slope and intercept
Plasma Concentration versus Time Plots
| kel, hr-1 | t1/2, hr | |
| Acetaminophen | 0.28 | 2.5 |
| Diazepam | 0.021 | 33 |
| Digoxin | 0.017 | 40 |
| Gentamicin | 0.35 | 2.1 |
| Lidocaine | 0.43 | 1.6 |
| Theophylline | 0.063 | 11 |
Copyright 2001 David W.A. Bourne




