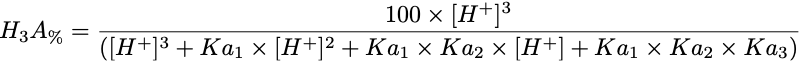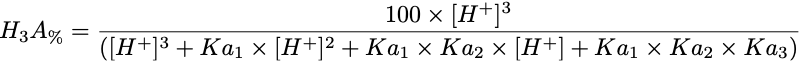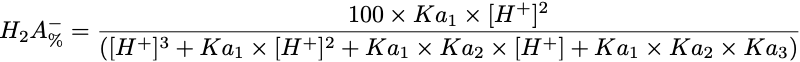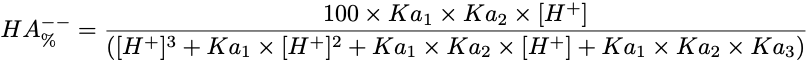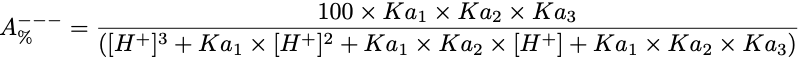Linear Plot of Species versus pH
Triprotic Weak Acid
Triprotic acids, such as citric or phosphoric acid, have three pKa values and thus four species at various pH values. These forms are the free acid form, H3A, and three salt forms, H2A-, HA-- and A---. The concentration of each species can be calculated with the equations.
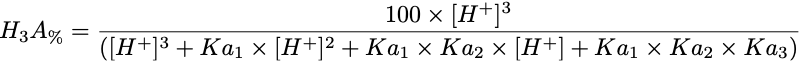
Equation 1 Fraction present as H3A
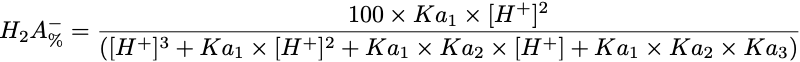
Equation 2 Fraction present as H2A-
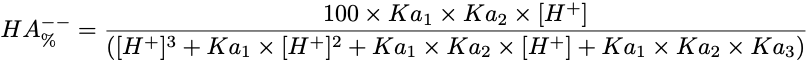
Equation 3 Fraction present as HA--
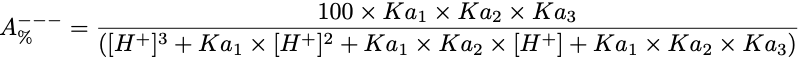
Equation 4 Fraction present as A---
Some Examples
| Acid |
pKa1 |
pKa2 |
pKa3 |
| Citric* |
3.15 |
4.78 |
6.40 |
| Phosphoric |
2.12 |
7.21 |
12.67 |
*Default for Graph above.
References
- Martin, A.N., Swarbrick, J. and Cammarata, A. 1970 Physical Pharmacy, 2nd Edition, Chapter 9 Ionic Equilbria, Tabel 9-1, Lea & Febiger, Philadelphia, PA