




Another important property of first order kinetics is the half-life of elimination, t1/2.
The half-life is the time taken for the plasma concentration to fall to half its original value. Thus if Cp = concentration at the start and Cp/2 is the concentration one half-life later then:-
![]()
![]()
ln 2 = kel * t1/2
or
![]()
OR
![]()
Note: Independent of concentration, a property of first order processes
These equations can be used as an approximate method of calculating kel. If we look at a plot of Cp versus time on semi-log graph paper.
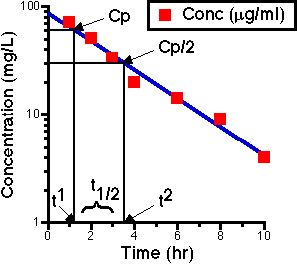
Figure IV-11. Semi-log Plot of Cp versus Time with Some Data and a Line
The steps to take
1) Draw a line through the points (this tends to average the data)
2) Pick any Cp and t1
3) Determine Cp/2 and t2
4) Calculate t1/2 as (t2 - t1)
Then kel = 0.693/t1/2
Also consider determining Cp/4 or Cp/8 for two half-lives or three half-lives, respectively
The line smooths out the bumps. There may be bad data points, so by putting in a line you 'sort of' average the data. The half-life is the same whether going from 40 to 20 or from 10 to 5 mg/L. This is a property of the first order process.
Note:
Go from:
Cp - > Cp/2 in 1 half-life i.e. 50.0 % lost 50.0 %
Cp - > Cp/4 in 2 half-lives i.e. 25.0 % lost 75.0 %
Cp - > Cp/8 in 3 half-lives i.e. 12.5 % lost 87.5 %
Cp - > Cp/16 in 4 half-lives i.e. 6.25 % lost 93.75 %
Cp - > Cp/32 in 5 half-lives i.e. 3.125 % lost 96.875 %
Cp - > Cp/64 in 6 half-lives i.e. 1.563 % lost 98.438 %
Cp - > Cp/128 in 7 half-lives i.e. 0.781 % lost 99.219 %
Thus over 95 % lost or eliminated in 5 half-lives. Typically considered the completion (my definition unless told otherwise) of the process, although in theory it takes an infinite time. Others may wish to wait 7 half-lives where over 99% of the process is complete.
See Table IV-1 from earlier, for some typical half-life values.
Copyright 2001 David W.A. Bourne




