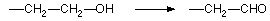Chapter 17
Metabolism
return to the Course index
previous | next
Metabolic Processes
Drugs may be metabolized by a wide variety of enzymes located throughout the body. Also, there is a wide variety of reactions that can be called metabolism. These reactions may be grouped into Phase 1 and Phase 2 type reactions. However, some have included Phase 0 and Phase 3 transport processes as part of the overall topic of metabolism.
Commonly there are four types of reactions involved in drug metabolism.
These are:
- oxidation
- reduction
- hydrolysis
----------
- conjugation
The first three are often lumped together as phase I reactions, while the fourth process, conjugation, is called phase II metabolism. A common scheme in the overall metabolism of drugs is that metabolites are metabolized. In particular a drug may be oxidized, reduced or hydrolyzed and then another group may be added in a conjugation step. A common cause of capacity limited metabolism is a limit in the amount of the conjugate added in the conjugation step.
Phase 0
Phase 0 has been described as the transport of drug from the blood into the heptacytes in the liver, the basolateral (sinusoidal) uptake processes (see Chapter 11) (Ishikawa, 1992).
Although not included in the Phase 0 designation, absorption of drugs from the intestinal lumen to the portal blood supply involves transport and metabolism enzyme processes. The reverse transport enzyme P-glycoprotein (PGP) is often accompanied by the metabolizing enzyme P450 3A (CYP3A). Both of these processes can significantly reduce drug bioavailability and provide a potential for drug interactions (Ritschel and Kearns, 2004).
Phase I
Phase 1 metabolic processes include oxidation, reduction and hydrolysis reactions which typically provide functional groups capable of undergoing Phase 2 reactions. The enzymes which catalyze Phase 1 reactions are found in a number of subcellular components including cytoplasm, mitochondria and endoplasmic reticulum. Although the liver is a major organ of metabolism, metabolic enzymes are found throughout the body.
Oxidation
Oxidation is the addition of oxygen and/or the removal of hydrogen. The cytochrome P450 enzymes are the most important of the oxidative enzymes. The cytochrome P450 or CYP family consists of a number of subfamilies such as CYP2C or CYP3A. The individual enzymes are numbered as CYP2C8 or CYP3A4. Hydroxylation is the introduction of an OH group by oxidation. The enzyme CYP3A4 is responsible for the oxidation of dapsone (N-hydroxylation), diazepam (3-hydroxylation), taxol (3'-hydroxylation), warfarin ((S)-4'-hydroxylation) and others. The enzyme CYP2D6 assists in the oxidation of alprenolol, amiodarone (aromatic hydroxylation), debrisoquine (4-hydroxylation), imipramine (2-hydroxylation), propranolol (4-hydroxylation), codeine (O-demethylation) and others. CYP2C9 is responsible for the oxidation of ibuprofen, phenytoin, tenoxicam, tolbutamide and warfarin (also CYP1A2).
A few example reactions
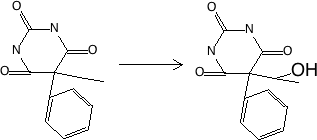
Figure 17.2.1 Aliphatic hydroxylation to alcohol - minor metabolite of phenobarbital
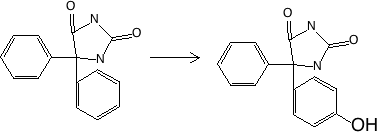
Figure 17.2.2 Aromatic hydroxylation to phenol - major metabolite of phenytoin, p-HPPH
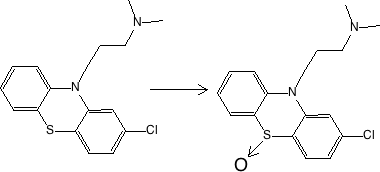
Figure 17.2.3 Oxidation at S (on N) - chlorpromazine to sulfoxide
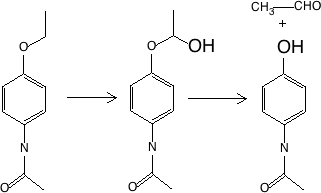
Figure 17.2.4 Two step oxidative dealkylation - phenacetin
Monoamineoxidaze
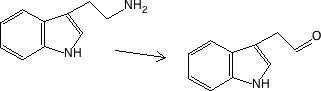
Figure 17.2.5 Oxidation - 5-hydroxytryptamine
Alcohol dehydrogenase - in liver, kidney, lung
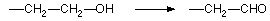
Reduction (add H or remove O)

Figure 17.2.7 Reduction of nitro to amine - nitrazepam
Hydrolysis
Addition of water with breakdown of molecule. In blood plasma (esterases) and
liver
Esters to alcohol and acid
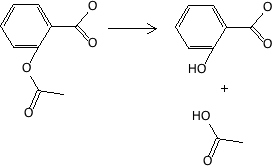
Figure 17.2.8 Hydrolysis - aspirin to salicylic acid (-OH) and acetic acid
Amides to amine and acid

Figure 17.2.8 Hydrolysis - procainamide to p-aminobenzoic acid
Phase 2
Conjugation
Conjugation reactions involve the addition of molecules naturally present in
the body to the drug molecule. The drug may have undergone a phase I reaction.
Glucuronidation
This is the main conjugation reaction in the body. This occurs in the liver.
Natural substrates are bilirubin and thyroxine. Aliphatic alcohols and phenols
are commonly conjugated with glucuronide. Thus hydroxylated metabolites can
also be conjugated. for example morphine
Acylation
Acylation, especially acetylation with the acetyl group, e.g. sulfonamides
Glycine
Glycine addition (NH2CH2COOH) for example nicotinic acid
Sulfate
Sulfate (-SO4) for example morphine, paracetamol
Phase 3
Elimination of the drug or metabolite into bile
Excretion by ATP dependent transporter (e.g. MRP2)
Metabolite is often more Polar
In most cases the metabolite is formed by production of a more polar group, for
example C-H -> C-OH, or addition of a polar group, for example acetyl (CH3COO-). Generally the resultant metabolite is more water soluble, and certainly less lipid soluble. Less drug is reabsorbed from the kidney.
Occasionally the metabolite is less water soluble. A significant example is the
acetyl metabolite of some of the sulfonamides. Some of the earlier sulfonamides
are acetylated to relatively insoluble metabolites which precipitated in urine,
crystalluria. The earlier answer this was the triple sulfa combination, now the
more commonly used sulfonamides have different elimination and solubility
properties and exhibit less problems.
Drug as a Pro-drug - Active Metabolite
In most cases the metabolites are inactive, however, occasionally the
metabolite is also active, even to the extent that the metabolite may be the
preferred compound to be administered. The original drug may take on the role
of a pro-drug.
For example:-
amitriptyline ---> nortriptyline
codeine ---> morphine
primidone ---> phenobarbital
Drug metabolism can be quantitatively altered by drug interactions. This
alteration can be an increase by induction of enzyme activity or a reduction by
competitive inhibition.
Pharmacogenomics - Pharmacogenetics
Pharmacogenomics and the older term pharmacogenetics describe the interaction between drug pharmacokinetics or activity and genetic or genomic parameters. While pharmacogenetics deals with genetic difference between individuals, pharmacogenomics deals with the more specific interaction with genes and single nucleotide polymorphisms (SNPs). Genetic polymorphism will cause differences in enzymes, proteins, transporters and receptors.
Responses to Pharmacogenomic Variation
- Alteration in enzyme activity may produce clinically significant differences in drug metabolism.
- Altered protein structure can cause altered drug protein binding
- Changes in drug transporters can alter drug absorption or distribution
- Drug receptor formation can be controlled genetically. Alterations in drug receptors may significantly change drug response.
Some definitions (from Wikipedia or the references below)
- Chromosomes consists of a long strand of deoxyribonucleic acid (DNA). All non reproductive human (diploid) cells contain two pairs of 22 chromosomes plus two sex determining chromosomes for a total of 46
- Each strand of DNA consists of a double chain of deoxyribose, pentose sugar, phosphate group and nitrogenous heterocyclic bases (adenine, cytosine, guanine or thymine). Specific sequences of base pairs in the DNA strands define the gene.
- a Gene is a specific section or location of the DNA strand of a chromosome that carries the coding information for some protein or structural RNA. It consists of at least forty base pairs. Genes take up only approximately 1% of a DNA strand of a chromosome. The cells transcribe the genetic information on the gene into RNA and the RNA is translated into a specific protein (enzymes, transporters or receptors). This process is called gene expression.
- Genetic or mutated variations in the base pair sequence in a gene are alleles also called polymorphism. Alleles are different forms of a gene. There may be numerous variations of alleles for any gene. The diploid cells contain two copies of each of the 22 non sex chromosomes. The 'normal' or more common allele is called the wild type. Modified or altered alleles may be called mutant. If the allele is copied one copy is inherited from the mother and from the father. If the alleles from each copy are the same the individual is homozygous for that genetic variation. Different alleles mean the individual is heterozygous. There may be many modifications or mutantant alleles to an particular gene. Some alleles may be dominant where only one copy is needed for a specific expressions. In other case the allele may be recessive and both copies must be the same (homozygous) for this gene expression. In other cases the heterozygous situation may provide an intermediate expression (result).
For example consider a gene that determines the activity of a particular enzyme. If the gene can exist as only two alleles (say A or B), with A being dominant, AA, AB, and BA will produce one (the more common) enzyme activity and only the BB case will provide the alternate enzyme activity. In other cases the two homozygous forms (AA and BB) will produce the extremes of enzyme activity and the heterozygous forms (AB and BA) will produce an intermediate activity. In other cases, there may be many more than two alleles so a wide range of enzyme (protein, transporter or receptor) may be expressed.
- Single nucleotide polymorphisms (SNPs) are alterations in a single base pair at a particular location on the DNA strand of a gene. SNPs may occur at any part of the DNA strand and commonly outside the ≈ 1% that include gene information.
A few examples
- The muscle relaxant succinylcholine is usually rapidly deactivated by plasma butyrylcholinesterase within a few minutes. However, in some individuals genetic variation in the expression of this enzyme results in reduced enzyme activity, reduced metabolism and prolong drug activity. Drug activity may last up to an hour in these individuals (Kalow, 2004).
- During World War II it was observed that some African-American soldiers suffered hemolytic toxicities after usual doses of the anti-malarial primaquine. This was later identified as a higher frequency of genetically controlled lack of the enzyme glucose-6-phosphate dehydrogenase (G6PD) (Kalow, 2004). In a MASH episode (210 - The Red/White Blues) Max Klinger (played by Jamie Farr), a regular character portraying a solder of eastern Mediterranean origin also exhibited symptoms of primaquine toxicity which was later attributed to a higher incidence of a genetic deficiency in this population as well.
- Fast and slow acetylators (N-acetyltransferase, NAT) of isoniazid have been identified in varying frequencies in different populations. Normal doses given to slow (unidentified) slow acetylators results in toxicities such as numbness, pain and tingling (Kalow, 2004).
- drug transporters, MDR1
- codeine metabolism to morphine - CYP 2D6
- warfarin dosing
Item 1. It is generally considered that much of the analgesic activity of codeine is due to one of its metabolites, morphine. The O-demethylation of codeine results in measurable, therapeutic concentrations of morphine. This pathway is enzymatically catalyze by CYP2D6 which has a number of genetically controlled alleles. Thus there are at least extensive (EM) and poor (PM) metabolizer of codeine. There are reports of intermediate and also poor intermediate metabolizers as well. PM produces almost no morphine and thus codeine is ineffective in these individuals. Thus, the concentration of morphine and its therapeutic efficacy is greatly reduced in PM. Explore this problem as a Linear Plot - Interactive graph.
References
- Kwon, Y. 2001 Handbook of Essential Pharmacokinetics, Pharmacodynamics and Drug Metabolism for Industrial Scientists, Chapter 8 Metabolism, Kluwer Academic, New York
- Ritschel, W.A. and Kearns, G.L. 2004 Handbook of Basic Pharmacokinetics ... including Clinical Applications, 6th ed., American Pharmaceutical Association, Washington, DC ISBN 1-58212-054-4
- Humma, L.M., Ellingrod, V.L. and Kolesar, J.M. 2003 Lexi-Comp's Pharmacogenomics Handbook, Lexi-Comp, Hudson, OH ISBN 1-59195-060-0
- Ishikawa T. 1992 The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci, pp463-468
- Licinio, J. and Wong, M.-L. 2002 Pharmacogenomics, Wiley-VCH, Weinheim, Germany ISBN 3-527-30380-4
- Vavricka, S.R. et al. 2002 Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver, Hepatology, Jul 36(1), pp164-72
- Kalow, W. 2004 Pharmacogenetics: A Historical Perspective in Pharmacogenomics: Application to Patient Care, Amer. College Clin. Pharmacy, Kansas, MO ISBN 1-880401-80-0
- P450 - Drug Table
- Pharmacogene Variation (PharmVar) Consortium
- FDA Guidance for Industry Pharmacogenomic Data Submissions (Mar 2005) and Companion Guidance (Aug 2007)
return to the Course index
This page was last modified: Sunday, 28th Jul 2024 at 5:00 pm
Privacy Statement - 25 May 2018
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | Pharmacy Math Part One
A selection of Pharmacy Math Problems |
 |