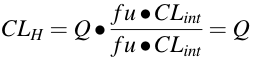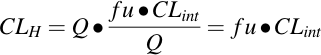Chapter 17
Metabolism
return to the Course index
previous | next
Hepatic Clearance
The systemic or total body clearance clearance, CL, is a measure of the efficiency with which a drug is irreversibly removed from the body. One important component of this total body clearance is liver or hepatic clearance, CLH. There are a number of models used to describe hepatic clearance including the venous equilibration model. This model include a number of parameters which can be considered in the understanding of hepatic clearance and liver disease or altered physiological state.
Venous equilibration model equation
We can consider the organ clearance as it may be measured in an isolated
organ system. Here we would have for example an isolated liver, perfused with
blood containing the drug of interest. By measuring the drug concentration in
the blood entering and leaving the organ at steady state, the organ clearance
can be measured directly for the drug.

Diagram 17.4.1 Blood Flow through the Liver
In Diagram 17.4.1, QH is the blood flow rate to the organ, CA is the concentration of drug in the blood entering the organ, and CV is the concentration of drug in the blood leaving the organ. The term E is the steady state extraction ratio. High E values mean high clearance by the liver and thus extensive metabolism.
The sum of the individual organ clearance values are equal to the systemic clearance, CL. For a drug which is eliminated entirely via the liver, the hepatic clearance is equal to the systemic or total body clearance. From the equation above we can see that the organ clearance is a function of the liver blood flow and the extraction ratio of the drug. The liver blood flow is a physiological parameter which may be altered in disease states. The extraction
ratio, we shall see shortly is a parameter dependent not only of the condition
of the liver but also the drug.
Both the hepatic clearance and the extraction ratio are empirical parameters
which can be used as measures of the efficiency of the elimination process.
They are dependent on three independent variables:-
- total hepatic blood flow (QH),
- fraction unbound (fU) or the extent of drug binding to blood constituents. This may be saturable with high dose, polar compounds, and
- the free intrinsic clearance (CLint) or the rate-limiting
step in drug uptake from blood, intracellular transport, metabolism, and where
necessary biliary secretion. The free intrinsic clearance may be thought of as
the clearance of drug from liver plasma water, devoid of the influence of blood
flow or binding. Since a major part of this parameter is metabolism which is
typically enzyme mediated this parameter may be saturated at higher doses, for
some drugs
The equation describing hepatic clearance in terms of these parameters using
the venous equilibration model can be defined as (Wilkinson and Shand 1975):-
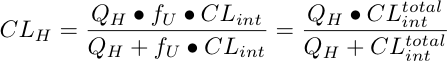
Equation 17.4.1 Hepatic Clearance
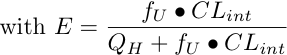
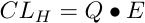
With this equation it is possible to look at the influence of free intrinsic
clearance, drug binding, and liver blood flow on the overall hepatic clearance
of a drug using applets calculating plasma concentrations after iv bolus or oral dosing.
Drugs can be classified into three types depending on the intrinsic
clearance and binding. Flow limited, capacity limited, and others.
Flow limited drugs
High fU • CLint (= CLtotalint) value. [fU • CLint >> QH]. For drugs with high total intrinsic clearance the extraction ratio, E, approaches 100%, the hepatic clearance approximates and is dependent of hepatic blood flow. Hepatic clearance is said
to be FLOW LIMITED. Also, we can note that the hepatic clearance is not
dependent on moderate changes in free intrinsic clearance or binding to blood
constituents.
Examples include:- lidocaine, propranolol, morphine.
Capacity limited drugs
Very low total intrinsic clearance. [fU • CLint << QH]. With drugs
having very low intrinsic clearance, hepatic extraction is inefficient and
hepatic clearance becomes independent of hepatic blood flow. Now changes in
free intrinsic clearance and/or binding to blood constituents becomes very
important in determination of the overall hepatic clearance. Hepatic clearance
is said to be CAPACITY LIMITED as the intrinsic capacity of the liver
controls the drug clearance.
Examples include:-
phenytoin, warfarin, and quinidine. For such drugs it is possible that liver
disease will cause a decrease in CLint but also an increase in fu. In this case
the overall hepatic clearance doesn't reflect just the hepatic metabolic
activity but also the drug binding. This is illustrated with tolbutamide. In
patients with hepatitis there is an increase in fu but no change in CLint. As a result CL is increased and the elimination half-life decreases. The change in elimination half-life reflects changes in binding and not changes in drug metabolizing activity.
Other drugs
Between these two extremes. Capacity-limited but binding-insensitive
drugs. The three parameters; QH, fU, and CLint are important determinants of drug elimination.
Examples include:- theophylline, antipyrine
There are other models for liver metabolism besides the well-stirred (venous equilbration) model described above, such as the parallel-tube (sinusoidal perfusion) and the dispersion model. Explore the well-stirred and parallel-tube models after IV administration and oral administration as interactive graphs.
As a reminder explore the relationship between extraction ratio, blood flow, clearance, apparent volume of distribution, half-life and elimination rate constant.

Figure 17.4.1 Clearance, V, kel and t1/2
Click on the figure to view the interactive graph
References
- Wilkinson, G.R., and Shand, D.G. 1975 A physiological approach to hepatic drug clearance, Clin. Pharmacol. Ther., 18, 377-90
return to the Course index
This page was last modified: Sunday, 28th Jul 2024 at 5:00 pm
Privacy Statement - 25 May 2018
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2025 David W. A. Bourne (david@boomer.org)
 | Catch the Sig
A game to aid in interpreting Prescription Sig instructions
See how many Sigs you can catch before you run out of lives |

|


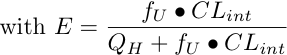
![]()