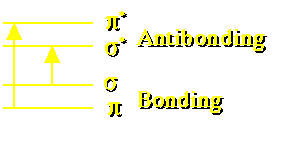
Figure 23.3.1 Sigma and Pi Bond Levels
return to the Course index
previous | next
Useful quantitation techniques include:
Most compounds include chromophores within their molecular structure. Thus these compounds absorb electromagnetic energy in the visible (350 - 700 nm) and/or ultraviolet range (200 - 350 nm). Within specific concentration ranges the amount of energy absorbed is proportional to the concentration of the compound in the sample.
A number of compounds which absorb light energy are also able to re-emit some of that energy as light at a higher wavelength (lower energy). The emitted energy can be measured and correlated with the concentration of the compound.
Radioactive atoms can be chemically incorporated into a compound of interest and subsequently used to quantitate the compound. The quality of the method depends on how well the radiolabel remains with the compound of interest.
Various compounds will undergo oxidation or reduction under the influence of an electrical potential. These electrochemical reactions result in an electrical charge which can be detected and used as a measure of the drug concentration.
Absorption by molecules in solution produces changes in electronic transitions as well as vibrational and rotational changes. For example the carbonyl group bonds contain sigma and pi electrons. These electrons may transition from bonding to antibonding levels.
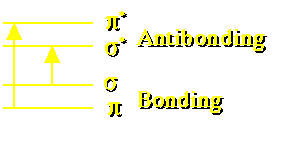
Figure 23.3.1 Sigma and Pi Bond Levels
Each of these transitions would result in a single peak in the absorbance / wavelength spectrum except for the broadening effect of the rotational and vibrational transitions.
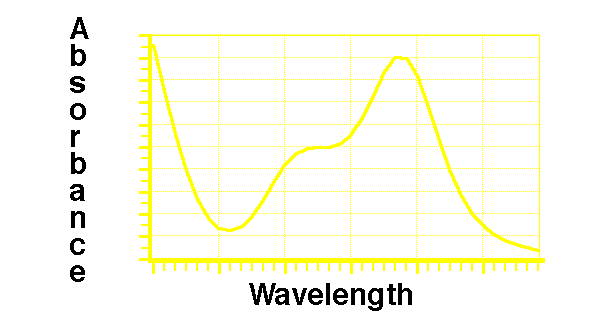
Figure 23.3.2 Plot of Absorbance versus Wavelength
As light passes through a compound in solution the intensity is reduced.
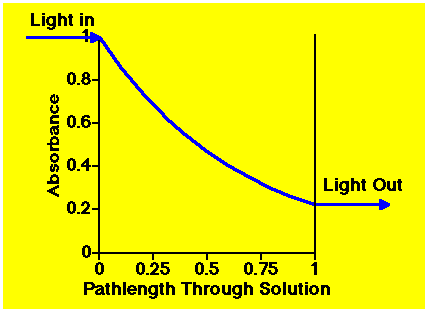
Figure 23.3.3 Light Absorbed through a Solution
The longer the pathlength the more light is absorbed. Also, the higher the concentration of compound in solution the more light is absorbed. Absorbance is proportional to pathlength and the concentration (Beer-Lambert's law)
Equation 23.3.1 Beer-Lambert Law for Light Absorption
where
a = absorptivity (ε, epsilon - molar absorptivity includes pathlength and wavelength)
b = pathlength (commonly 1 cm)
c = concentration (molar if molar absorptivity)
If b is 1 cm and c is in g/100ml the absorptivity is given as A1%1 cm at wavelength (lambda).
Absorptivity may also be called the extinction coefficient or absorption coefficient
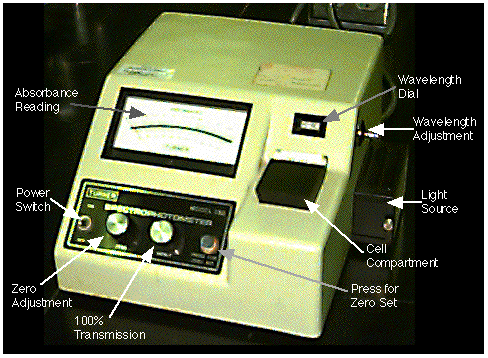
Figure 23.3.4 Turner Model 330 Spectrophotometer
The Turner model 330 single beam spectrophotometer has a cell holder for the sample and dials for zero adjustment, 100% transmission and wavelength. Absorbance is read from the upper scale on the meter.

Figure 23.3.5 Schematic of a Double Beam Spectrophotometer
Redrawn from: Bauer, H.H., Christian, G.D., and O'Reilly, J.E. 1978 Instrumental Analysis, Figure 7.14, page 187
Analysis of Drugs by Visible Spectroscopy

Figure 23.3.6 Absorbance versus Concentration
One Compartment Model - IV Bolus - Multiple Dose
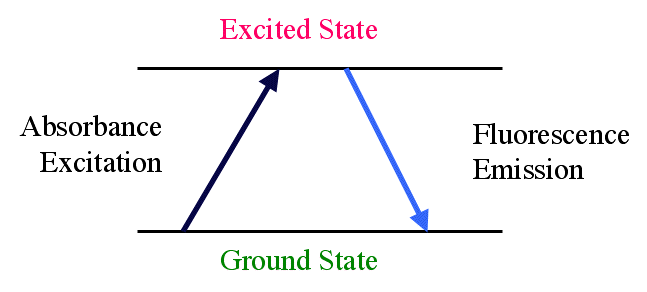
Fig 23.3.7 Absorption to an Excited Energy State followed Fluorescence Emission

Fig 23.3.8 Schematic of a Fluorescence Detector
Redrawn from: Instrumental Analysis by Bauer, Christian and O'Reilly, 1978, page 235
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | A game to aid recognizing brand versus generic drug names See how many names you can catch before you run out of lives |
|