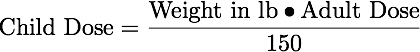
Equation 24.3.1 Clarks' Rule
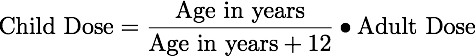
Equation 24.3.2 Young's Rule
return to the Course index
previous | next
Further complications are that little data is available concerning the disposition of drugs in infants before the general release of new drugs. Also studies in infants and young children are difficult to perform because of the limited amount of sample which can be collected. The most dramatic changes occur in the first year. For children older than one year, dose adjustments can often be made on a weight or surface area basis without too many problems.
Extravascular fluid is proportionately higher at an earlier age as well. In general distribution volumes expressed as volume per body weight tend to be larger in neonates than in adults and decrease towards adult values during childhood. This has been observed for ampicillin, ticarcillin, and amikacin. Binding to plasma proteins appears to be less in newborn infants compared with older children and adults. This appears to be true for both acidic and basic drugs. The presence of competing substances, such as bilirubin in premature infants, complicates the picture.
Caffeine is very slowly metabolized in newborns. During the first month almost no metabolism occurs, with half-lives of about 4 days resulting from renal elimination, normally a minor pathway. Between 3 and 7 months, caffeine is metabolized similarly to adults and the half-lives change to adult values during this period. For the similar compound theophylline the half-life was 13 to 29 hours for 8 low birth weight infants. See Hale (2004) p112-3.
Glucuronidation is quite inefficient at birth, thus chloramphenicol which is normally glucuronidated in adults and has no major alternate metabolic pathway, the overall elimination is much slower in newborns compared with adults.
Sulfate conjugation is well developed at birth thus newborn acetaminophen elimination, predominantly sulfation, is not greatly different from that of adult elimination.
For drugs which undergo M-M or saturable metabolism the effect of age is interesting. For phenytoin, Km is not changed with age, but the maximum metabolism rate, Vm falls progressively with younger patients.
| Age | GFR (ml/min/m2) |
|---|---|
| First four days | 1 |
| 14 days | 22 |
| One year | 70 |
| Adult | 70 |
Renal tubular capacity, measured by renal clearance of p -aminohippurate, achieve adult values 1 to 2 months later. Therefore drugs which depend primarily on the renal route of elimination, such as gentamicin, ampicillin, and furosemide, have prolonged elimination times in neonates and young infants.
| Neonate | Adult | |
|---|---|---|
| Gastric acid output (mEq/10kg/hr) | 0.15 | 2 |
| Gastric emptying time (min) | 87 | 65 |
| Total body water (% of body weight) | 78 | 60 |
| Extracellular water (% of b.wt.) | 44 | 19 |
| Intracellular water (% of b.wt.) | 34 | 41 |
| Adipose tissue (% of b.wt.) | 12 | 12-25 |
| Serum albumin (gm/dL) | 3.7 | 4.5 |
| Glomerular filtration rate (ml/min/m2) | 11 | 70 |
| Age group | Volume term (L/kg) |
Half-life (hr) |
Total body clearance (ml/min/kg) |
|---|---|---|---|
| Theophylline | |||
| Premature neonates | 0.62 (0.19-1.0) |
26.9 (14.4-57.7) |
19 (6.3-29.9) |
| Infants | 0.44 (0.16-0.83) |
4.6 (0.8-8.6) |
76 (28-156) |
| Children | 0.44 (0.20-0.68) |
3.4 (1.9-8.5) |
95 (60-221) |
| Adults | 0.47 (0.33-0.72) |
5.7 (2.9-8.3) |
65 (32-131) |
| Gentamicin | Vc | CL (ml/min/1.73 m2) | |
| Preterm infants and full-term infants | 0.48 | 5.7 | 21.0 |
| Infants and children | 0.28 | 1.4 | 130 |
| Adults | 0.21 | 2.1 | 95 |
| Chloramphenicol | Vd | CL (ml/hr/kg) | |
| Infants (11-56 d) | 10 | ||
| Infants (1-12 mo) | 0.90 | 5.5 | 50-400 |
| Children (1-11 yr) | 0.90 | 4.4 | 100-400 |
| Adults | 0.4-0.9 | 2-5 | 100-300 |
For older children minimal adjustments can be based on weight, surface body area, or age.
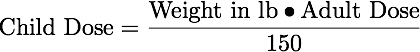
Equation 24.3.1 Clarks' Rule
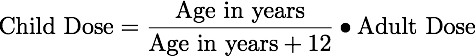
Equation 24.3.2 Young's Rule
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | A game to aid in interpreting Prescription Sig instructions See how many Sigs you can catch before you run out of lives |
|