
Diagram XI-2, the Davson-Danielli Model





Diagram XI-2, the Davson-Danielli Model
These results suggest that the biologic membrane is mainly lipid in nature but contains small aqueous channels or pores. Other experiments involving surface tension measurements have suggested that there is also a layer of protein on the membrane. These results and others have been incorporated into a general model for the biological membrane. This is the Davson-Danielli model.
The membrane then acts as a lipid barrier with small holes throughout.
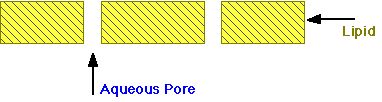
Diagram XI-3, Simplified Model of Membrane
This is the general structure. Membranes in different parts of the body have somewhat different characteristics which influence drug action and distribution. In particular, pore size and pore distribution is not uniform between different parts of the body.
Examples of some membrane types.
Blood-brain barrier. The membranes between the blood and brain have effectively no pores. This will prevent many polar materials (often toxic materials) from entering the brain. However, smaller lipid materials or lipid soluble materials, such as diethyl ether, halothane, can easily enter the brain. These compounds are used as general anesthetics.
Renal tubules. In the kidney there are a number of regions important for drug elimination. In the tubules drugs may be reabsorbed. However, because the membranes are relatively non-porous, only lipid compounds or non-ionized species (dependent of pH and pKa) are reabsorbed.
Blood capillaries and renal glomerular membranes. These membranes are quite porous allowing non-polar and polar molecules (up to a fairly large size, just below that of albumin, M.Wt 69,000) to pass through. This is especially useful in the kidney since it allows excretion of polar (drug and waste compounds) substances.
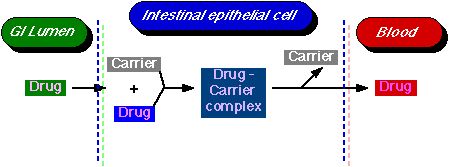
Diagram XI-4, Carrier-Mediated Transport Process
The body has a number of specialized mechanisms for transporting particular compounds; for example, glucose and amino acids. Sometimes drugs can participate in this process; e.g. 5-fluorouracil. Active transport requires a carrier molecule and a form of energy.
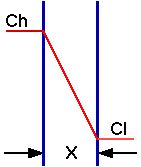
Diagram XI-5, Diagram of Passive Transport with a Concentration Gradient
Most drugs cross biologic membranes by passive diffusion. Diffusion occurs when the drug concentration on one side of the membrane is higher than that on the other side. Drug diffuses across the membrane in an attempt to equalize the drug concentration on both sides of the membrane.
If the drug partitions into the lipid membrane a concentration gradient can be established.
The rate of transport of drug across the membrane can be described by Fick's first law of diffusion:-
Rate of diffusion =
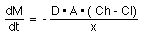
Equation XI-1 Rate of Diffusion
The parameters of this equation are:-
D: diffusion coefficient. This parameter is related to the size and lipid solubility of the drug and the viscosity of the diffusion medium, the membrane. As lipid solubility increases or molecular size decreases then D increases and thus dM/dt also increases.
A: surface area. As the surface area increases the rate of diffusion also increase. The surface of the intestinal lining (with villae and microvillae) is much larger than the stomach. This is one reason absorption is generally faster from the intestine compared with absorption from the stomach.
x: membrane thickness. The smaller the membrane thickness the quicker the diffusion process. As one example, the membrane in the lung is quite thin thus inhalation absorption can be quite rapid.
(Ch -Cl): concentration difference. Since V, the apparent volume of distribution, is at least four liters and often much higher the drug concentration in blood or plasma will be quite low compared with the concentration in the GI tract. It is this concentration gradient which allows the rapid complete absorption of many drug substances.
Normally Cl << Ch then:-
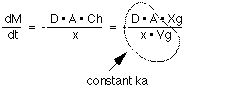
Thus the absorption of many drugs from the G-I tract can often appear to be first-order.
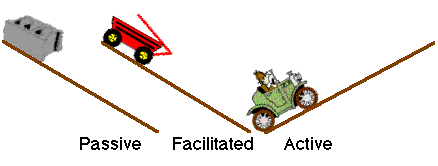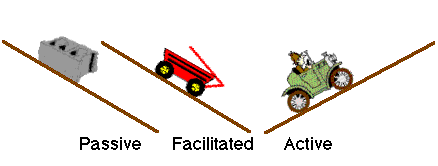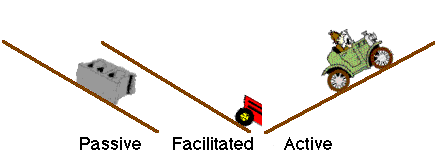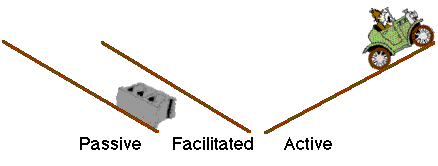
Diagram XI-6, Illustration of Different Transport Mechanisms
Copyright 2001 David W.A. Bourne



