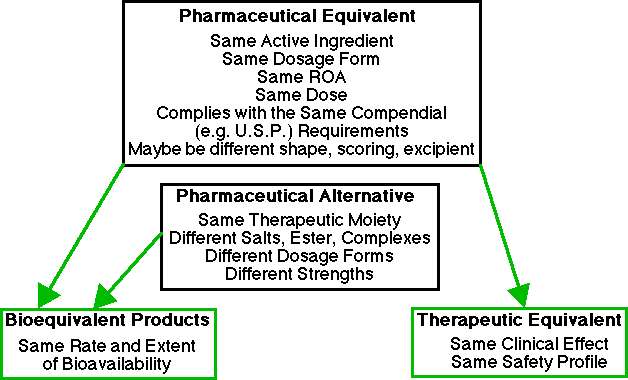
Figure 10.2.1 Summary of Bioavailability DEfinitions
return to the Course index
previous | next
Pharmaceutical Equivalent "Drug products are considered pharmaceutical equivalents if they contain the same active ingredient(s), are of the same dosage form, route of administration and are identical in strength or concentration (e.g., chlordiazepoxide hydrochloride, 5mg capsules). Pharmaceutically equivalent drug products are formulated to contain the same amount of active ingredient in the same dosage form and to meet the same or compendial or other applicable standards (i.e., strength, quality, purity, and identity), but they may differ in characteristics such as shape, scoring configuration, release mechanisms, packaging, excipients (including colors, flavors, preservatives), expiration time, and, within certain limits, labeling."
Pharmaceutical equivalents are the same drug entity, the same type of dosage form, the same dose and meet the same compendial requirements. For example Aspirin Tablets, U.S.P. of a particular strength. Although the U.S.P. monograph includes dissolution and chemical assay requirements there are no bioavailability requirements (at least not in U.S.P. XX). Thus all Aspirin U.S.P. tablets of a particular dose would be pharmaceutical equivalents. Capsules of aspirin would not and neither would tablets of a different dose. Dosage forms containing different salt forms, esters or other chemical form are not pharmaceutical equivalents.
Pharmaceutical Alternatives "Drug products are considered pharmaceutical alternatives if they contain the same therapeutic moiety, but are different salts, esters, or complexes of that moiety, or are different dosage forms or strengths (e.g., tetracycline hydrochloride, 250mg capsules vs. tetracycline phosphate complex, 250mg capsules; quinidine sulfate, 200mg tablets vs. quinidine sulfate, 200mg capsules). Data are generally not available for FDA to make the determination of tablet to capsule bioequivalence. Different dosage forms and strengths within a product line by a single manufacturer are thus pharmaceutical alternatives, as are extended-release products when compared with immediate- or standard-release formulations of the same active ingredient."
Pharmaceutical alternatives are drug products that can provide the same therapeutic moiety. Different dosage forms, doses and even salts can be pharmaceutical alternatives.
Therapeutic Equivalent "Drug products are considered to be therapeutic equivalents only if they are pharmaceutical equivalents and if they can be expected to have the same clinical effect and safety profile when administered to patients under the conditions specified in the labeling."
Thus, pharmaceutical equivalents that have been shown to be bioequivalent (and the same by other determinations of clinical effect and safety profile) are therapeutic equivalents. Therapeutic equivalents would be expected to produce identical drug concentration time profiles and therapeutic response when administered under the same conditions. This is not the same as two pharmacologically similar (equivalent) compounds that may produce the same therapeutic response in some individuals ("e.g., propoxyphene hydrochloride vs. pentazocine hydrochloride for the treatment of pain").
Bioequivalent Drug Products "This term describes pharmaceutical equivalent or alternative products that display comparable bioavailability when studied under similar experimental conditions. Section 505 (j)(7)(B) of the Act describes one set of conditions under which a test and reference listed drug shall be considered bioequivalent:
the rate and extent of absorption of the test drug do not show a significant difference from the rate and extent of absorption of the reference drug when administered at the same molar dose of the therapeutic ingredient under similar experimental conditions in either a single dose or multiple doses; or the extent of absorption of the test drug does not show a significant difference from the extent of absorption of the reference drug when administered at the same molar dose of the therapeutic ingredient under similar experimental conditions in either a single dose or multiple doses and the difference from the reference drug in the rate of absorption of the drug is intentional, is reflected in its proposed labeling, is not essential to the attainment of effective body drug concentrations on chronic use, and is considered medically insignificant for the drug. |
Where these above methods are not applicable (e.g., for drug products that are not intended to be absorbed into the bloodstream), other in vivo or in vitro test methods to demonstrate bioequivalence may be appropriate."
Bioequivalence Requirement [Code of Federal Register] means a requirement imposed by the Food and Drug Administration for the in vitro and/or in vivo testing of specified drug products which must be satisfied as a condition of marketing.

Figure 10.2.1 Summary of Bioavailability DEfinitions
Brand Name [Shargel and Yu, 1985] is the trade name of the drug.
Chemical Name [Shargel and Yu, 1985] is the name used by the organic chemist to indicate the chemical structure of the drug. The IUPAC Name.
Drug Product [Federal Register 1977] means a finished dosage form, e.g., tablet, capsule, or solution, that contains the active drug ingredient, generally, but not necessarily, in association with inactive ingredients.
Generic Name [Shargel and Yu, 1985] is the established, non proprietary or common name of the active drug in a drug product.

Figure 10.2.2 A Collection of Names for the Same Drug
Brand names may be obsolete, taken from Billups, 1986
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | A game to aid recognizing drug structures See how many structures you can name before you run out of lives |
|