
Equation 4.10.1

Equation 4.10.2

Equation 4.10.3
![]()
Equation 4.10.4
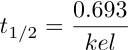
Equation 4.10.5 |
OR | 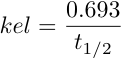
Equation 4.10.6 |
return to the Course index
previous | next
Another important property of first order kinetics is the half-life of elimination, t1/2.

Equation 4.10.1

Equation 4.10.2

Equation 4.10.3
![]()
Equation 4.10.4
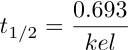
Equation 4.10.5 |
OR | 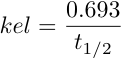
Equation 4.10.6 |
Note: Independent of concentration. This a property of first order processes
These equations can be used as an approximate method of calculating kel. If we look at a plot of Cp versus time on semi-log graph paper.

Figure 4.10.1 Semi-log Plot of Cp versus Time Illustrating t1/2 Calculation
The steps to take are:
And finally kel = 0.693/t1/2 (Equation 4.10.5)
You might also consider determining Cp/4 or Cp/8 after two half-lives or three half-lives, respectively. This should provide a more accurate answer as the differences in Cp and t will be larger.
The line smooths out the bumps. There may be less accurate data points, so by putting in a line you average the data. The half-life is the same whether going from 40 to 20 or from 10 to 5 mg/L. This is a property of the first order process.
Note:
Go from:
Cp - > Cp/2 in 1 half-life i.e. 50.0 % lost 50.0 %
Cp - > Cp/4 in 2 half-lives i.e. 25.0 % lost 75.0 %
Cp - > Cp/8 in 3 half-lives i.e. 12.5 % lost 87.5 %
Cp - > Cp/16 in 4 half-lives i.e. 6.25 % lost 93.75 %
Cp - > Cp/32 in 5 half-lives i.e. 3.125 % lost 96.875 %
Cp - > Cp/64 in 6 half-lives i.e. 1.563 % lost 98.438 %
Cp - > Cp/128 in 7 half-lives i.e. 0.781 % lost 99.219 %
Thus over 95 % is lost or eliminated after 5 half-lives. Typically, with pharmacokinetic processes, this is considered the completion (my definition unless told otherwise) of the process [Although in theory it takes an infinite time]. Others may wish to wait 7 half-lives where over 99% of the process is complete. Others have suggested that three half-lives are sufficient.
| Drug | t1/2, hr |
| Acetaminophen | 2.5 |
| Diazepam | 33 |
| Digoxin | 43 |
| Gentamicin | 2 |
| Lidocaine | 1.8 |
| Theophylline | 5.5 |
In the pharmacokinetic area of study the half-life of a drug usually refers to the biological or terminal half-life. These terms have different meaning for some people. I tend to view them both as referring to half-life measured for the terminal or slowest slope on the semi-log drug concentration versus time plot. At low concentration more processes tend to follow first order kinetics. However, at later times with lower concentrations assay sensitivity can be a serious problem. Also, if absorption is very slow the slowest slope may refer to the absorption process instead of drug disposition.
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | A game to aid recognizing drug structures See how many structures you can name before you run out of lives |
|