
Equation 9.5.1 Drug Concentration versus Time after Oral Administration
return to the Course index
previous | next
Returning to the equation for Cp as a function of time

Equation 9.5.1 Drug Concentration versus Time after Oral Administration
We can calculate ka and kel given Cp versus time data. From the method of residuals, the intercept can be determined as
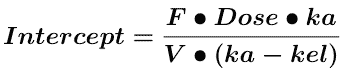
Since we know the dose and have calculated ka and kel, it is possible to calculate F/V. However, with only data from a single oral administration available that is all we can determine; we cannot separate V and F. Of course if we have IV data for kel and V, we could use this to determine F.
Thus F must be determined by comparison with another dose administration. If the other dosage form is an intravenous dose then the F value is termed the absolute bioavailability. In the case where the reference dosage form is another oral or other non IV product , the value for F is termed the relative bioavailability.
When a bioavailability study is conducted at least two dosage forms are administered to each subject. One dosage form is the product to be tested, while the other dosage form is a standard or reference dosage form. This may be an IV dose, oral solution or most commonly the original manufacturer's product. The doses are given with sufficient time between administrations for the drug to "washout" or be completely eliminated. We usually assume that each subject eliminates each dosage form at similar rates or use the estimate of the slowest rate to determine the wash-out period.
During the derivation of the Wagner-Nelson equations we calculated Amax, the maximum amount absorbed as:-

Equation 9.5.2 Amax, Total Amount Absorbed
or
![]()
and since
![]()

Equation 9.5.3 Bioavailability or Fraction Absorbed
Now by giving two dosage forms A and B, and calculating AUC values for each we can calculate the relative bioavailability of dosage form A with respect to dosage form B, FA/FB.

Equation 9.5.4 Bioavailability of Product A Relative to Product B
and if we can assume that kelA = kelB and VA = VB then

Equation 9.5.5 Bioavailability, F, from AUC Comparison
Thus a relative bioavailability, F, can be calculated. If dosage form B is an IV administration then FB = 1 and F = FA and thus FA represents the absolute bioavailability.

Since

Equation 9.5.6 Fraction Excreted as Unchanged Drug, fe
therefore

and for two dosage forms

if we assume feA = feB then



Equation 9.5.8 Calculation of F from IV and PO AUC values

Equation 9.5.9 Calculation of F from V and V/F
The last step after the calculation of absorption rate constant, ka, using the method of residuals involves the calculation of F using Equation 9.5.9.
return to the Course index
previous | next
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | Pharmacy Math Part One A selection of Pharmacy Math Problems |
 |