
Equation 12.2.1 Brodie's D Value
return to the Course index
previous | next
Brodie et al. (Shore, et al. 1957) proposed the pH - partition theory to explain the influence of GI pH and drug pKa on the extent of drug transfer or drug absorption. Brodie reasoned that when a drug is ionized it will not be able to get through the lipid membrane, but only when it is non ionized and therefore has a higher lipid solubility.
Brodie tested this theory by perfusing the stomach or intestine of rats, in situ, and injected the drug intravenously. He varied the concentration of drug in the GI tract until there was no net transfer of drug across the lining of the GI tract. He could then determined the ratio, D as:

Equation 12.2.1 Brodie's D Value
i.e.

Equation 12.2.2 Brodie's D Value in Another Form

Figure 12.2.1 Diagram Showing Transfer Across Membrane
These values were determined experimentally, but we should be able to calculate a theoretical value if we assume that only non ionized drug crosses the membrane and that net transfer stops when [U]b = [U]g
Brodie found an excellent correlation between the calculated D value and the experimentally determined values.
where HA is the weak acid and A- is the salt or conjugate base

Equation 12.2.3 Dissociation Constant - Weak Acids
taking the negative log of both sides
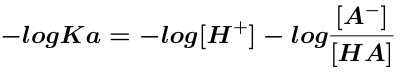
and rearranging gives Equation 12.2.4 where pKa = -log Ka and pH = -log[H+]

Equation 12.2.4 Henderson - Hasselbach Equation - Weak Acids
where B is the weak base and HB+ is the salt or conjugate acid

Equation 12.2.5 Dissociation Constant - Weak Acids
taking the negative log of both sides

and rearranging gives Equation 12.2.6 where pKa = -log Ka and pH = -log[H+]

Equation 12.2.6 Henderson - Hasselbach Equation - Weak Bases
or alternately

Equation 12.2.7 Dissociation Constant - Weak Base
taking the negative log of both sides


Note that Ka • Kb = [H3O+] • [OH-] = Kw which is approximately 10-14, thus pKb - pOH = pH - pKa and the equation above can be changed into Equation 12.2.6.
| Weak Acid | pKa |
| Acetic | 4.76 |
| Acetylsalicyclic | 3.49 |
| Ascorbic | 4.3, 11.8 |
| Boric | 9.24 |
| Penicillin V | 2.73 |
| Phenytoin | 8.1 |
| Salicyclic | 2.97 |
| Sulfathiazole | 7.12 |
| Tetracycline | 3.3, 7.68, 9.69 |
| Weak Base | pKb |
| Ammonia | 4.76 |
| Atropine | 4.35 |
| Caffeine | 10.4, 13.4 |
| Codeine | 5.8 |
| Erythromycin | 5.2 |
| Morphine | 6.13 |
| Pilocarpine | 7.2, 12.7 |
| Quinine | 6.0, 9.89 |
| Tolbutamide | 8.7 |

Figure 12.2.2 Diagram Illustrating Drug Distribution between Stomach and Blood (Weak Acid)
Compare D for a weak acid (pKa = 5.4) from the stomach (pH 3.4) or intestine (pH 6.4), with blood pH = 7.4

Equation 12.2.8 Weak Acid from Stomach

Equation 12.2.9 Weak Acid from Blood
Therefore the calculated D value would be


Equation 12.2.10 Brodie D Value - Weak Acid (Stomach)

Figure 12.2.3 Diagram Illustrating Drug Distribution between Intestine and Blood (Weak Acid)
By comparison in the intestine, pH = 6.4
The calculated D value is (100+1)/(10+1) = 9.2
From this example we could expect significant absorption of weak acids from the stomach compared with from the intestine. Remember however that the surface area of the intestine is much larger than the stomach. However, this approach can be used to compare a series of similar compounds with different pKa values.

Figure 12.2.4 Plot of ka versus fu
With this theory it should be possible to predict that by changing the pH of the G-I tract that we would change the fraction non ionized and therefore the rate of absorption.
Thus kaobserved = ku • fu assuming that the ionized species is not absorbed.

Figure 12.2.5 Plot of ka (apparent) versus fu for Sulfaethidole from Rat Stomach
Redrawn from Crouthamel, W.G., Tan, G.H., Dittert, L.W. and Dolusio, J.T.
1971 Drug absorption IV. Influence of pH on absorption kinetics
of weakly acidic drugs, J. Pharm. Sci., 60, 1160-63
For some drugs it has been found that the intercept is not zero in the above plot, suggesting that the ionic form is also absorbed. For example, results for sulfaethidole. Maybe the ions are transported by a carrier which blocks the charge, a facilitated transport process.
 Monoprotic Species versus pH |
 Biprotic Species versus pH |
 Triprotic Species versus pH |
 pH Rate Profile |
return to the Course index
previous | next
Material on this website should be used for Educational or Self-Study Purposes Only
Copyright © 2001 - 2026 David W. A. Bourne (david@boomer.org)
 | A game to aid in interpreting Prescription Sig instructions See how many Sigs you can catch before you run out of lives |
|